Patient is a 77 year old man with history of HTN, hyperlipidemia, former smoking, and CAD with CABGx5 and bilateral lower extremity bypasses who developed unstable angina consisting of neck and throat pain. He underwent catheterization at an outside hospital and found to have 100% LAD occlusion, a diseased, small patent left main and left circumflex (the profunda femoral artery of the heart!), 100% RCA occlusion, a patent but diseased SVG to distal RCA, and a patent LIMA graft to distal LAD but with severe plaque and near occlusion of his proximal left subclavian artery.

He had an NSTEMI. His vitals signs stabilized in the coronary care unit and he was sent to a telemetry floor. Whenever he walked, he would get the jaw pain, and this would also occur sporadically while recumbent.
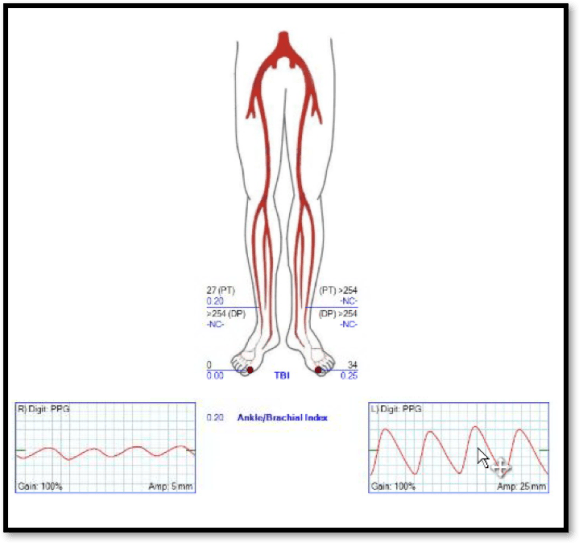
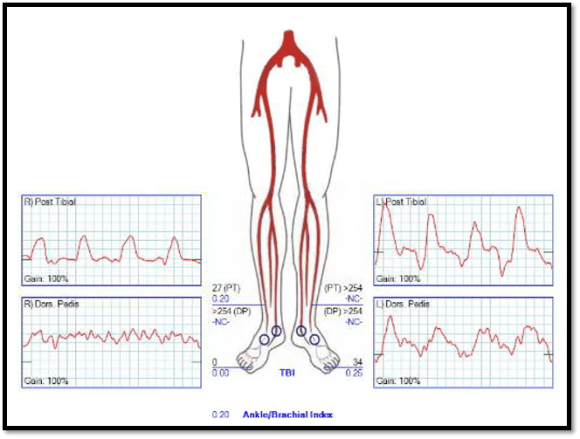
On examination, he had no left brachial pulse, only a monophonic signal there, and bounding femoral pulses where there were the origins of bilateral femoral-tibial bypasses. His radial artery pulse was diminished on the right and absent on the left. Both saphenous veins had been harvested as were arm veins for the left leg bypass.
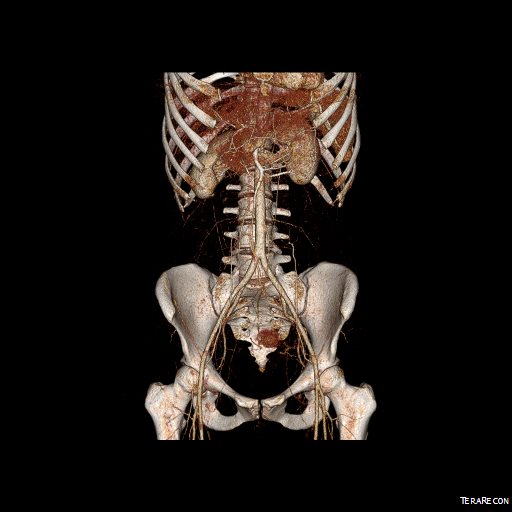
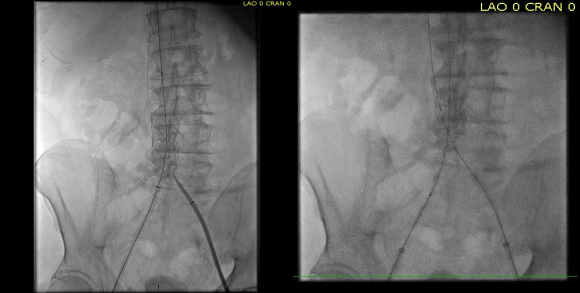
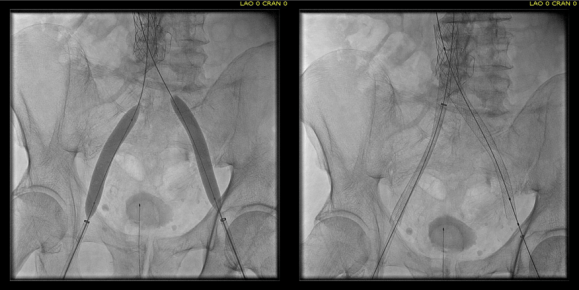
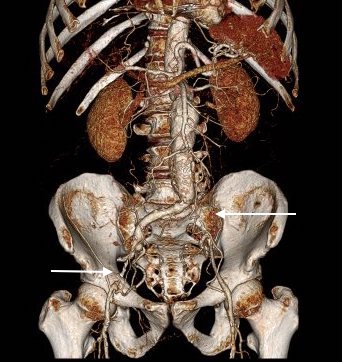
CTA shows the left subclavian artery to be occluded at its origin.


Cardiac surgery, interventional cardiology, and vascular surgery were called in for consultation. Cardiology consultation (Drs. Kapadia and Shisheboor) felt, and I agreed, that the left subclavian lesion was a poor candidate for recanalization and stenting. CT Surgery (Dr. Faisal Bakaeen) and I had a long discussion regarding alternate conduits, as he had unknown radial but likely radial artery disease, and had all usable veins previously harvested. I brought up a free RIMA graft -I had worked with Dr. Daniel Swistel, in NYC as a resident, who was Dr. George Green’s protege, and as a medical student at P&S I scrubbed Dr. Green’s final cardiac case. He routinely performed bilateral ITA bypasses decades before all-arterial revascularizations were routine. I get enthusiastic talking about cardiac disease! Walking through all the options -does anyone use deep femoral vein as coronary bypass conduit -we agreed ultimately that the best option would be a carotid-subclavian bypass with plenty of backup.

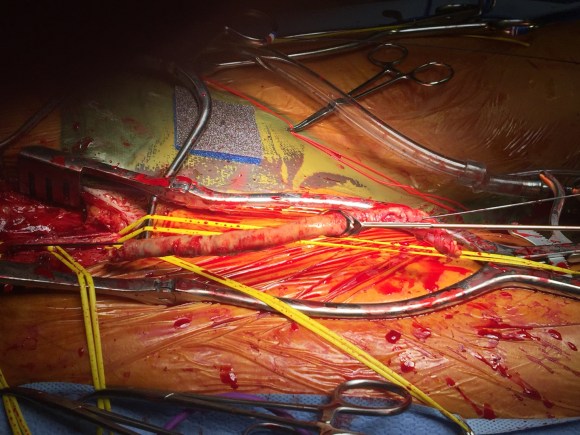
At its heart, it would be this vascular surgeon’s attempt at an off-pump single vessel CABG (above). Preparations were made with cardiac anesthesia and cardiac surgery to place an IABP (intra-aortic balloon pump) if he became unstable. For my part, the operation was straightforward, but I was going to have to go about it efficiently. I also figured that with a clamp beyond the LIMA takeoff, no significant change would occur to the coronary flow from the LIMA graft. So I hoped as I worked very deliberately. We kept him on the hypertensive side during the case.
The operation went well. The patient’s angina resolved and a followup CT showed the patent bypass feeding the LIMA and LAD.
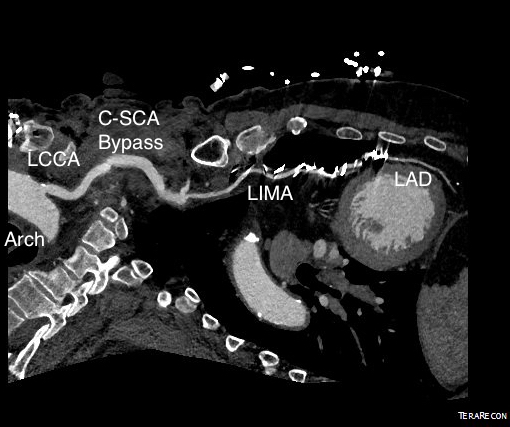

His resting angina resolved. He followed up a month later and was very pleased. Moreover, he had a brachial and radial artery pulse and a general weakness of the left arm that he never complained about before lifted.
Discussion
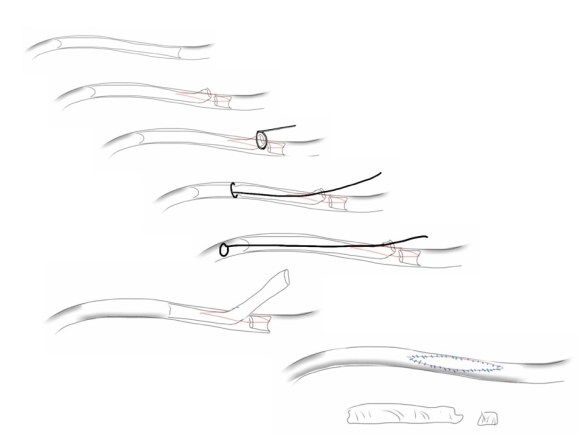
The carotid subclavian bypass is something that really needs to be in the armamentarium of a modern vascular surgeon. Though out of print, Wylie’s Atlas (the unabridged, multivolume version) is available used through online sellers, and is useful for elucidating the anatomy which boils down to avoiding cutting the important structures -the phrenic nerve, the vagus nerve, the brachial plexus, branches of the subclavian including the vertebral artery, while cutting away muscles -lateral head of sternocleidomastoid, any part of the omohyoid, the anterior scalene muscle. And dividing the lymphatic duct if encountered. And tunneling under the jugular vein. And minding the buttery fragility of the SCA. The best technical paper out there is by Dr. Mark Morasch and it mostly deals with carotid-subclavian transposition (reference 1) but has excellent figures on bypass as well. I do both transposition and bypass, but for brevity, I prefer bypass.
This is not a unique problem, having been reported in the literature. An unusual variant of this is coronary sbuclavian steal syndrome (reference 2), which refers to reversal of flow in the LIMA bypass in the setting of subclavian artery occlusion and left arm exertion -which was not the case here, but interesting enough to mention. Here, it was a straightforward case of managing the hemodynamics. The key point of operating on such a patient was having the surety of quick response in the case of ischemic heart failure -we operated in the cardiovascular operating rooms with rows of perfusion pumps and balloon pumps and VADs and ECMOs at the ready. Indeed, this result could not have been so straightforward and routine seeming without the combined effort and experience of the whole Heart and Vascular Institute from nursing to consultant staff.
Reference
- Morasch MD. Technique for subclavian to carotid transposition, tips, and tricks. J Vasc Surg 2009;49:251-4.
- Cua B et al. Review of coronary subclavian steal syndrome. J Cardiol. 2017 Apr 14. pii: S0914-5087(17)30090-4. doi: 10.1016/j.jjcc.2017.02.012. [Epub ahead of print]






















 The inflow graft area is taken from its measured diameter. Then usually one or the other artery has an obligate size -a size the graft has to be while the other has more “wiggle room.” The other thing that comes from experience is that the AFX graft’s iliac limb extension don’t get the B-infolding that can affect an oversized stent graft placed in a small artery and it accomodates a neighbor well.
The inflow graft area is taken from its measured diameter. Then usually one or the other artery has an obligate size -a size the graft has to be while the other has more “wiggle room.” The other thing that comes from experience is that the AFX graft’s iliac limb extension don’t get the B-infolding that can affect an oversized stent graft placed in a small artery and it accomodates a neighbor well.