Thoracoabdominal stent grafts off the shelf: link
Congratulations to Dr. Gustavo Oderich who has implanted the Gore off the shelf TAAA device at Mayo.
Thoracoabdominal stent grafts off the shelf: link
Congratulations to Dr. Gustavo Oderich who has implanted the Gore off the shelf TAAA device at Mayo.
At usual location. Topic renal and mesenteric vascular disease. Presenting are:
Dr. Michael O’Neil – Symplicity HTN3
Dr. Daniel Scott –Reop OR Mesenteric Ischemia CCF-Mayo
Dr. Lynsey Rangel –Open v Endo Mesenteric Ischemia

The first patient, a man in his late 70’s, ruptured in the emergency room at around four in the afternoon on a weekday, which was fortuitous, as the hospital was fully staffed, fully armed. The patient had arrived only a bit earlier with the complaint of severe abdominal pain, and soon after getting his CT, arrested. CPR commenced as I arrived by Dr. Timothy Ryan, our chief resident at that time.

More recently, while I was finishing up two urgent cases, I got a call that the patient with the leaking aneurysm had arrived from across town and was becoming hypotensive.




Ruptured aortic aneurysms are the sine qua non of vascular surgical practice. As a junior resident back in the antedeluvian 90’s, I remember one of my chiefs, Dr. Eric Toschlog, now a trauma surgeon out East, running a patient upstairs from the ER with a rupture, and before the attending arrived by taxi, had the graft in. When it became my turn, as a fellow working on a patient who had been helicoptered in from the frozen wastes of Minnesota, I remember playing a trick with my mind -that the patient was proportionally the same size as the rabbits I was working with in the research lab, that I was really big and the patient’s aneurysm very small. This works to calm the heart, steady the hand. Nowadays, my mind is blank, and my hands working reflexively.
There has been a series of papers that create scores that allow prediction of odds for survival, and both of these patients, particularly with their prolonged CPR, have greater than 90% predicted mortality on these measures. In this month’s JVS, Broos et al, in the aptly named paper, “A ruptured abdominal aortic aneurysm that requires preoperative cardiopulmonary resuscitation is not necessarily lethal” describe a 38.5% survival rate among their series of patients with rupture who had CPR (ref 1).
Practically speaking, no one I know would use these scores to decide to not operate. While many series show better survival for emergency EVAR compared to open repair, several randomized control trial failed to show better results when these methods were directly compared. A retroperitoneal approach is preferred by some in our group, but having tried both closed chest CPR with the patient in right lateral decubitus position and open cardiac massage -(both died), I prefer supine.
There is no survival if there is no attempt.
Reference

The patient, now in his 90’s, found out about his aortic and iliac artery aneurysms in his early 80’s, had been offered repair, but had refused. Several years ago, one of my partners emergently repaired his ruptured AAA (abdominal aortic aneurysm) via a retroperitoneal approach using a tube graft. At the time of the repair of the AAA, the common iliac artery aneurysms (CIAA’s) were not ruptured and would have added risky time to the repair. He survived and had a postop CT done about two years ago which showed his CIAA’s.

He was recently admitted for treatment of another condition, when his physicians noted a large visible pulsatile mass on his lower abdomen.

A CT scan was performed. It showed a 13 cm left common iliac artery aneurysm which was responsible for the visible puslatile mass and a large right common iliac artery aneurysm. The left internal iliac artery was thrombosed. His right common iliac artery aneurysm was over 5cm in size.

My partner, Dr. Ezequiel Parodi, was consulted for this case. He performed a percutaneous EVAR. The procedure was made difficult by tortuosity in iliac artery and the tube graft in the aorta requiring a secondary access from the arm to straighten out and advance the stent graft (aka body floss).

In followup, the aneurysms decreased in size and showed no endoleak around a patent stent graft.

Common iliac artery aneurysms expand at a rate proportional to their starting size and have increased rates of expansion in those with prior aortic aneurysm expansion (ref 1). Rupture probably signals a tendency to expand rapidly. There is evidence that iliac ectasia and aneurysms left over after tube graft repair (aorto-aortic) of AAA is benign and can be safely observed (ref 2), but these were all small at the start.
I had been trained at the dusk of the open surgical era and the dictum was aortobi-iliac bypasses to avoid future problems with the iliac arteries. With EVAR, and soon bifurcated iliac branched stent-grafts (currently on trial), staged repair of metachronous iliac aneurysms after tube graft repair of AAA has not only an appeal, but some logic as open bypass to iliac bifurcations, particularly in large men, is challenging and potentially morbid. This is a case of a patient who had a large iliac aneurysm that was not repaired initially due to the exigencies of ruptured AAA and had refused planned staged repair. His aneurysm grew from over 5cm to 13cm in 2 years time without rupturing. Such good fortune is very rare.
Vascular surgeons like to collect large aneurysm stories like fishermen talk about big fish. This is the largest unruptured common iliac artery aneurysm I have seen. While it is baffling to many who are in healthcare, it is important to understand noncompliance is common. Denial is a powerful urge, and a uniquely human one.

References
Sometimes the best conduit is no conduit.
The patient presented with abdominal pain and fevers which was initially diagnosed as a urinary tract infection. He is an older man with a prosthetic aortic valve and prostatic cancer who had a Foley catheter for several weeks leading up to a prostatectomy. Antibiotics relieved his abdominal pain. Echocardiogram revealed aortic valve vegetations. A CT scan revealed a mycotic mesenteric aneurysm and vascular surgery was consulted.

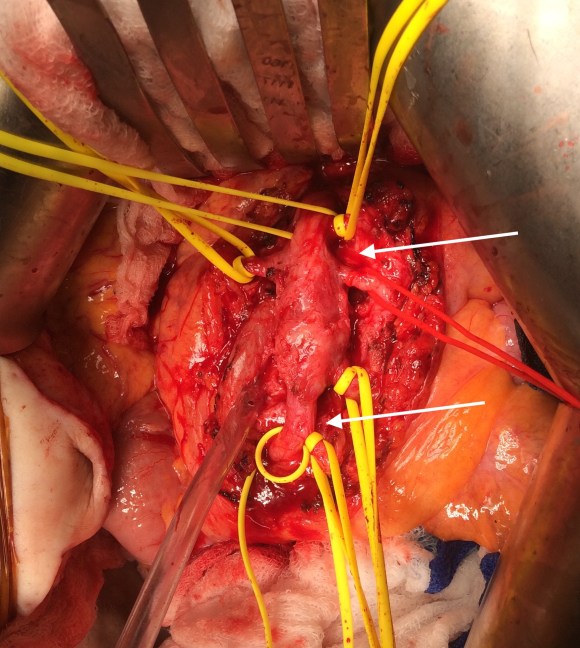
Examination revealed moderate cachexia and a soft abdomen. He was taken to the operating room for resection of aneurysm. Laparotomy revealed a mass in the small bowel mesentery root. The aneurysm had moderate but not excessive amounts of inflammation.
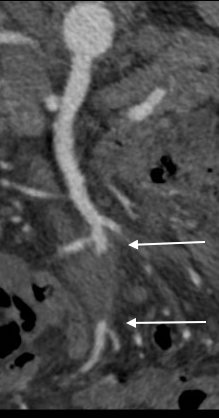
The CTA had shown the superior mesenteric artery to be patent above and below what was likely an embolized vegetation (see white arrows in all images).
The treatment goal was aneurysm resection with an intraoperative determination of need for revascularization. His thighs were prepped for possible saphenous vein harvest and cryopreserved artery was available.
Resection revealed the artery to be infected. There was good backbleeding from the distal SMA.
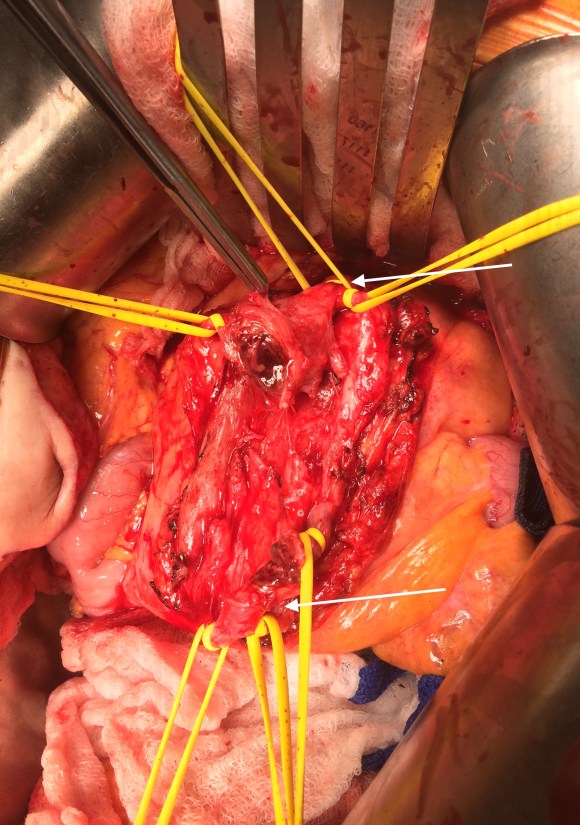
The handheld Doppler signals in the distal segment of SMA were excellent, corresponding to the viable bowel, but the patient’s cachexia and relative inanition concerned me for future bowel ischemia complicating his planned redo aortic valve replacement and subsequent prostatectomy.
But before I embarked on vein harvest, a simple maneuver determined my next step. I brought the distal SMA to the proximal SMA and found there was enough laxity to simply anastomose both to each other.
 The flows were now excellent in the SMA. The patient recovered uneventfully, requiring no subsequent bowel resection. He then had his redo-aortic valve replacement from which he recovered well from and ultimately soon after had his prostatectomy.
The flows were now excellent in the SMA. The patient recovered uneventfully, requiring no subsequent bowel resection. He then had his redo-aortic valve replacement from which he recovered well from and ultimately soon after had his prostatectomy.
Treatment with antibiotics without resection is not a good option as the majority of these rare aneurysm go on to rupture if left unresected. A frequently cited article by Drs. DeBakey and Cooley from 1953 (ref 1) and other subsequent articles show success with simple resection. While cryografts and saphenous vein grafts are subject to infection, they can be used safely in this setting, but the best conduit using no conduit. Often, aneurysms start at a small nidus and not only expand but elongate, given an opportunity to repair aneurysms primarily.
Reference

Type B aortic dissections (TBAD) are frequently seen here at the Clinic as we serve as a regional referral center. As a trainee, I read the chapters discussing all the classifications and discussions of the biomechanics and felt quite intimidated by the all the moving parts involved in an aortic dissection, and I missed the main point about TBAD. Aside from the rupture risk due to the attenuation of the adventitia and hypertension, the acute TBAD is a rapidly developing stenosis of the aorta due to the inflation of a wind sock balloon created by the dissection flap. You can assume any flow that occurs in the false lumen is limited by the area of the proximal tear which is always smaller than the area of the aortic lumen. The true lumen is still perfusing the lower half of the body, and because of the volume filling effect of the flap, flow is restricted. The equivalent physiology is seen in aortic coarctation. Long term, the false lumen behaves like a pseudoaneurysm and may thrombose, continue to grow, or both.
Our group looked at CT’scans on 80 consecutive patients and found that the true lumen to false lumen ratio of less than 0.37 is predictive of the need for intervention.

This makes hemodynamic sense as it approximates the 70% critical stenosis borderline for other arteries. It explains why closing the opening of the dissection, the opening of the wind sock, and expanding the true lumen effectively treats malperfusion.

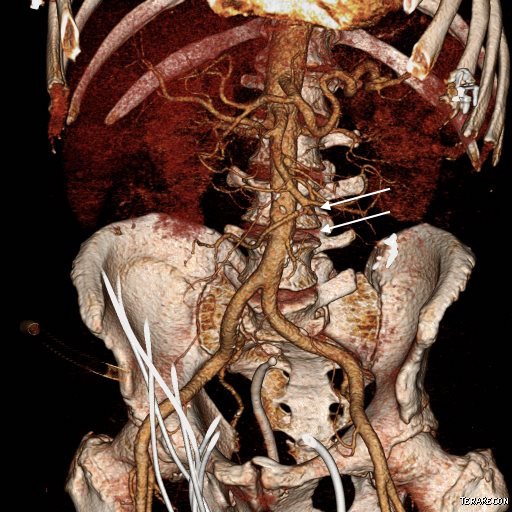
This patient whose CTA is shown above was transferred with increasing abdominal pain, inability to control blood pressure, and worsening lactic acidosis.

There was nearly complete obliteration of the true lumen throughout the aorta and occlusion of the left renal artery and dissection into the celiac and superior mesenteric arteries.

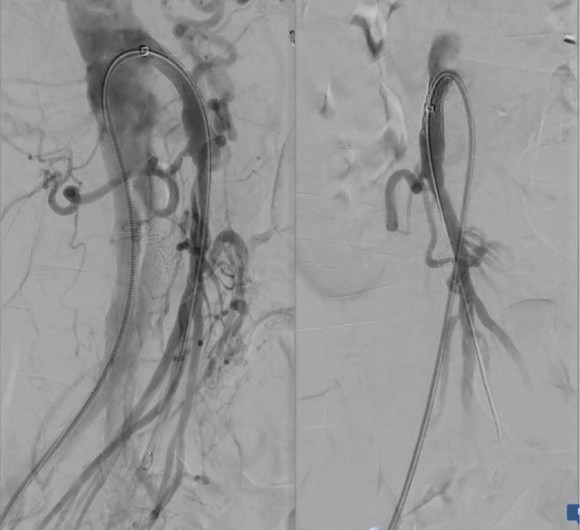
Aortography showed the dissection, and absence of visceral vessels from the injection which was from the aortic root. True lumen position was confirmed with IVUS.

A thoracic stent graft was delivered across the left subclavian artery origin up to the innominate artery origin -the patient had a bovine arch. Immediately, there was filling of the visceral vessels with re-establishment of true lumen flow.

The renal occlusion appeared improved but there was still a stenosis due to deflated dissection flap and this was stented (panel right above).
His abdominal pain remitted and his lactate normalized. His creatinine stabilized and has since normalized.
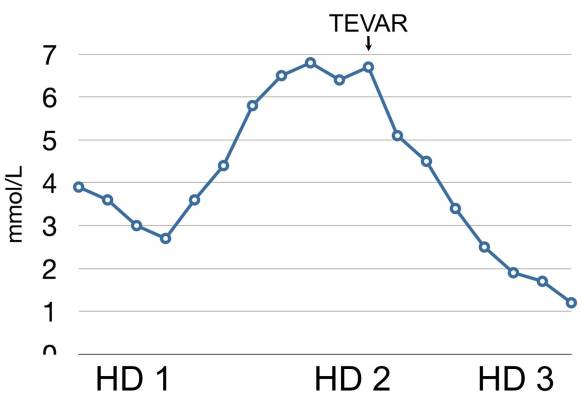
Again, if the true-lumen is compressed, the aorta is stenotic because there is a wind sock inflated in it. TEVAR offers a minimally invasive option, frequently percutaneous, for treating this.

The patient presented with complaints of leg and foot pain with sitting and short distance calf claudication, being unable to walk more than 100 feet. This is unusual because sitting usually relieves ischemic rest pain. He is in late middle age and developed claudication a year prior to presentation that was treated with stent grafting of his superficial femoral artery from its origin to Hunter’s canal at his local hospital. This relieved his claudication only briefly, but when the pain recurred a few months after treatment, it was far worse than what he had originally. Now, when he sat at his desk, his foot would go numb very quickly and he would have to lie down to relieve his pain.
On examination, the patient was moderately obese with overhanging belly. He had a palpable right femoral pulse, but nothing below was palpable. He had multiphasic signals in the dorsalis pedis and posterior tibial arteries. The left leg had a normal arterial exam. Pulse volume recording and segmental pressures were measured:

These are taken with the patient lying down which was the position that relieved his pain, and the PVR’s show some diminishment of inflow. It would be easy at this point to declare the patient’s pain to be due to neuropathy or spinal stenosis, but because of his inability to walk more than a hundred feet and because of his severe pain with sitting, I went ahead and obtained a CTA.
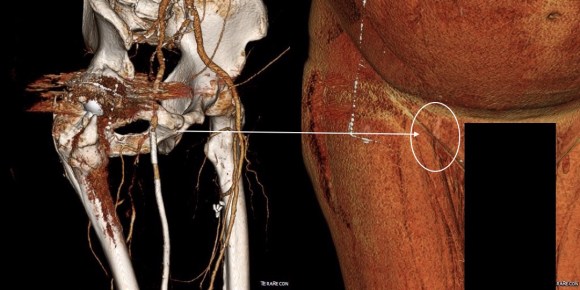
The CTA showed he had an occluded superficial femoral artery (SFA) with patent profunda femoral artery (PFA) with reconstitution of an above knee popliteal artery with multivessel runoff. The 3DVR image showed his inguinal crease to be right over the femoral bifurcation which is not an unsual finding, but his stent graft was partially occluding his profunda femoral artery.
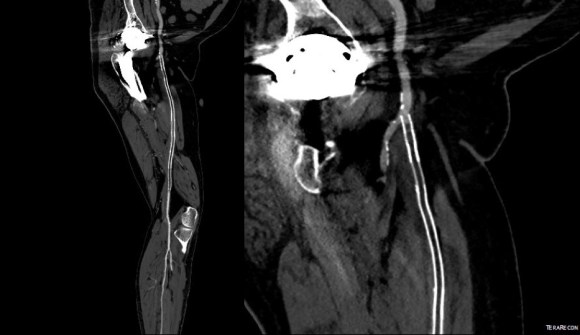
I decided to take him to the operating room to relieve his PFA of this obstruction. My plan was to remove the stent graft at the origin of the SFA and at the same time, remove the plaque and occluded stent graft from his SFA to restore it to patency.
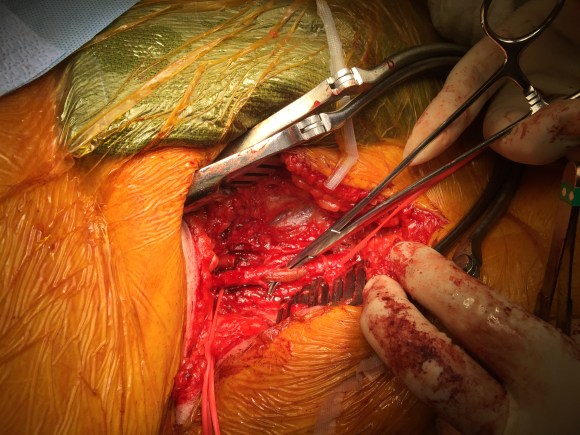
In the OR, on exposing his SFA, I discovered that because of his overhanging belly, his inguinal ligament had sagged and was compressing his femoral bifurcation.

This explained his presentation. The stent graft really had no chance as when he sat, the belly and ligament compressed it at the origin, and because it partially occluded the origin of the PFA, sitting probably pinched off flow completely. The 3dVR image shows the mid segment of PFA to have little contrast density -this is not because of thrombus, but because of the obstruction, the PFA was getting collateral flow from the hypogastric artery.
The stent graft was removed at its origin via a longitudinal arteriotomy after remote endarterectomy of the distal graft.
In this case, the Multitool (LeMaitre) was useful in dissecting the plaque and stent graft because of its relatively stiff shaft compared to the standard Vollmer rings. The technique of EndoRE has been described in prior posts (link).
The stent graft came out in a single segment -they come out easier than bare stents.


 The patient regained palpable pulses in his right foot and recovered well, being discharged home after a 4 day stay.
The patient regained palpable pulses in his right foot and recovered well, being discharged home after a 4 day stay.
While one could argue that just taking out the short piece of occlusive stent graft over the PFA was all that was necessary, I feel that there is no added harm in sending down a dissector around the stent, and in this patient there was restoration of his SFA patency which was the intent of the original procedure.
Unlike PTFE bypasses that sometimes fail with thromboembolism, SFA EndoRE fails with development of focal stenoses. From a conversation I had with Dr. Frans Moll at the VEITH meeting, I found that he has had good experience with using drug coated balloons in the treatment of these recurrent stenoses.
At the time of discharge, the patient was relieved of his rest pain, and was no longer claudicating. The common femoral artery, its bifurcation, and the profunda femoral artery remain resistent to attempts at endovascular treatment, and remain in the domain of open surgery. And in retrospect, the history and physical examination had all the clues to the eventual answer to the oddities of the patient’s complaints. The combination of inguinal crease, abdominal pannus, and low hanging inguinal ligament meant these structures acted to crush the stent graft and femoral bifurcation.

The patient was referred to me after having undergone an intervention for chronic mesenteric ischemia. She is over 70 years of age and had lost over thirty pounds in 3 months due to severe abdominal pain with eating. A month prior to seeing me, she had undergone arteriography at an outside hospital and was found to have occlusion of her celiac axis (CA) and superior mesenteric artery (SMA) with a small but patent inferior mesenteric artery. Attempt at recanalization, done from left brachial access, of the SMA was abandoned after the patient started having pain, and the inferior mesenteric artery was accessed and stented with a balloon expandable stent. Despite the stent, the pain persisted. On examination, she was cachectic, weighing about a hundred pounds, and had moderate to severe pain with abdominal palpation. CT angiography (shown above) showed a chronically occluded CA and SMA and a patent stent to the IMA.
After discussion with the patient about the possibility of a bypass, we decided to proceed with diagnostic arteriography and an attempt at recanalization. When planning these, I always try to come from the groin first as most of the time I am able to revascularize from below. I try to avoid 6F sheaths in the arms of thin cachectic patients -women especially where the brachial artery is likely the same diameter as a 6F sheath. The only downside about coming from below is that it is technically challenging and the stent comes off at a higher angle than the SMA typically has in situ.
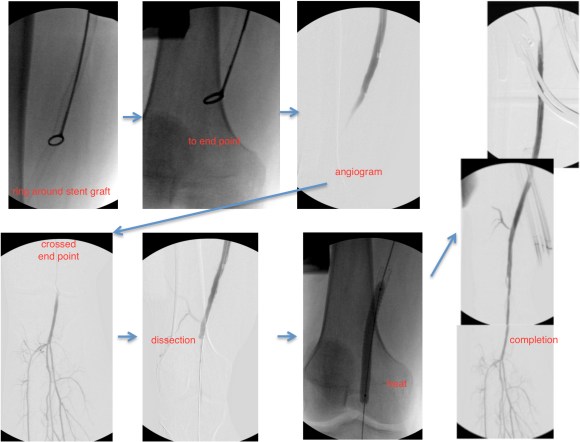
The image below shows the procedure:

The series of images shows the initial aortogram and access. The superior mesenteric artery has a stenosis at the origin, with an area of post stenotic dilatation or small aneurysm, which occludes beyond the first three branches of the SMA. The IMA fails to feed the bowel -the later phases not shown shows filling from the SMA segment to the CA and ileal branches.
The key step to this procedure is in getting “deep” access with a wire -in this case a floppy Glidewire, which I used to cross the occluded SMA. There is feedback from the tip which occurs if you spin it without a torque device. The wire has the quality, a feature really, of being tacky when dry, allowing for a great deal of coaxial spin with your first two fingers and your thumb. The tip transmits information about what it is crossing as you spin it -this is something that is hard to teach at first, but is gained largely through experience, but I learned it from Dan Clair over a decade ago when he barked at me to get rid of the torque device (“a tool for babies!”). The tip will go where it should if you spin, not push.
Once the wire is buried, a suitable catheter that tracks well is brought across the occlusion. Again, while there are many catheters that can do this, the Glide Catheter is suitable again from spinning across an occlusion over the wire that would push out the lowest profile and equally hyrdophillic catheters. Once the catheter is buried, a suitably stiff wire (in this case a Rosen wire) should be brought across -this widens the arc created by the wire as it goes up and over the SMA origin and allows for delivery balloons and stents. Using the balloon-piton technique (a requisite for FEVAR), the sheath is brought into the SMA, securing access into it.
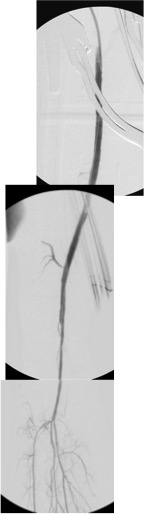
The occlusion in the mid-SMA ballooned nicely and did not require a stent -a nonocclusive dissection is seen but I chose not to treat this as placing a stent is likely to cause as many problems as solve and the dissection is in line with flow. The origin was stented with a balloon expandable stent -having the patient awake is useful in determining if the stent is “big enough.” Final arteriography in two planes is shows below.

Gratifyingly, the entire mesenteric system in the CA (foregut) and SMA (midgut) lit up. I admitted her for observation as I have seen patients develop bowel infarction with reperfusion which may be due to embolization but I think just as likely due to edema. Food needs to be reintroduced slowly as there maybe metabolic consequences to rapid refeeding. Her baseline lactate was 2.6mMol/L but came down to 0.8mMol/L the next day. Her other labs were normal. Her pain remitted and she was able to tolerate a regular diet by postoperative day 2.
Discussion:
Mesenteric ischemia is a particularly morbid condition. When it presents acutely, there is a high mortality rate (ref 1). Revascularization in good risk individuals is still bypass surgery (ref 2,3). The inferior mesenteric artery offers a dismal revascularization target for this reason -while the artery will remodel and dilated in the setting of mesenteric ischemia, its orifice from the aorta does not and is usually no more than 1-2mm from birth to adulthood. Also, while the large bowel will get perfusion from the IMA, and the foregut may get collateral flow from collaterals fed from the middle colic via the Arc of Riolan, the midgut does not get sufficient flow from from the IMA because it requires the longest path to fill the ileal and jejunal branches. The development of atherosclerosis in the aorta further complicates attempts at stenting. Despite this, it is still attempted (ref 4) and in 4 patients was successful at relieving pain for short periods of time, with one patient requiring eventual bypass despite characterization as “high risk.” It is a reflection of how poorly this vessel does with intervention that this 4 case series is the largest in the literature.
The analogy to IMA stenting in the legs is stenting of a heavily diseased profunda femoral artery in the setting of critical limb ischemia with femoropopliteal occlusive disease. It is occasionally successful in the short term, but will only delay the inevitable operation. There are no low risk patients with severe weight loss due to mesenteric ischemia. Aggressive intervention offers a path of survival for these patients, and but long term results are only possible with bypass.
References
December 15, 2015, usual place. Topic, femoropopliteal occlusive disease.
Dr. H. El-Arousy: 1-s2.0-S0741521415000646-main (Viabahn antiplatelet v anticoagulation)
Dr. J. Rowse: Circulation-2015-Krankenberg-CIRCULATIONAHA.115.017364 (Drug coated balloon v standard balloon in stent restenosis)
Dr. F. Vargas: 1-s2.0-S0735109713014149-main (1) (Drug eluting stents)
The patient had undergone EVAR for bilateral common iliac artery aneurysm with the original Gore Excluder stent graft a dozen years before with coil embolization and extension to the external iliac on the larger side and femoral to internal iliac artery bypass on the other side. A coagulopathy, one of the clotting factor deficiencies, had made him high risk for bleeding with major open surgery. His aneurysms never shrank but remained stable and without visible endoleak by CT for a long time resulting in ever longer intervals between followup.

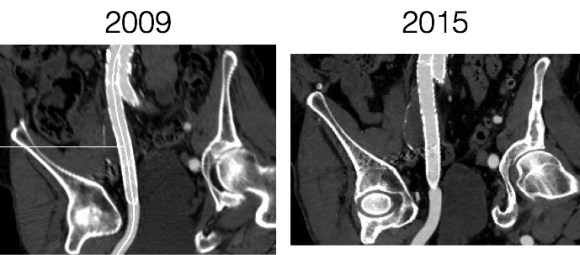
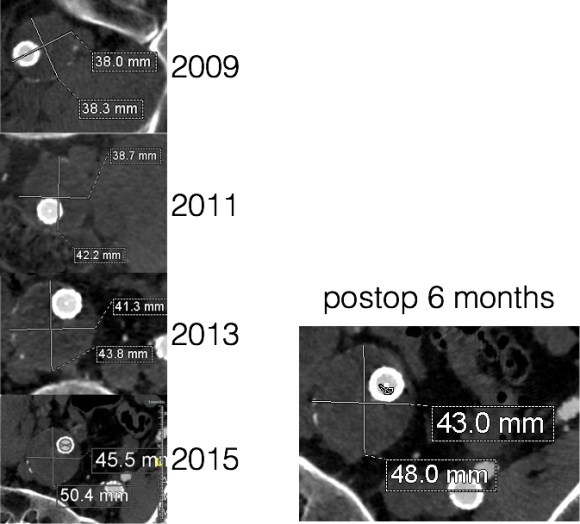
Between 2009 and 2013, there was subtle enlargement on the embolized side without a type I or type III leak, and the patient was brought back a year and a half later, with further growth of the sac.
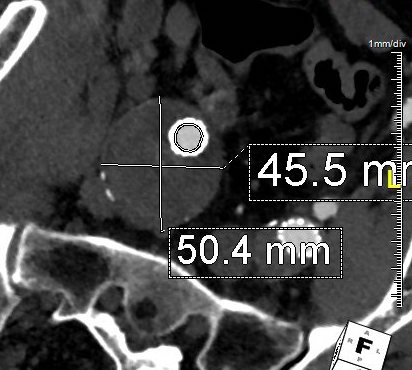
This was a relatively rare type IV endoleak that was causing sac enlargement due to excessive graft porosity of the original Excluder’s graft material. Its treatment is either explantation or relining. We chose to reline the graft with an Excluder aortic cuff at the top and two Excluder iliac limbs.
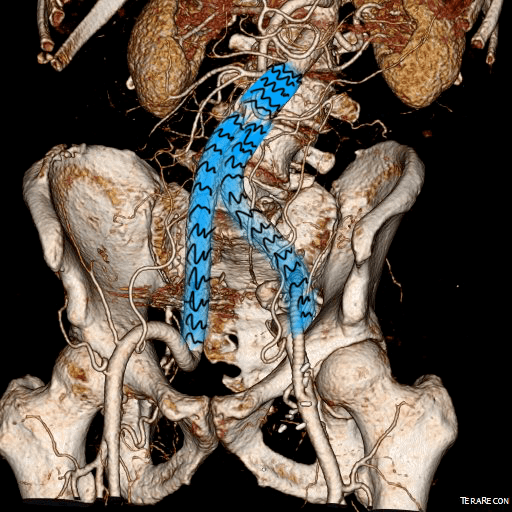
This was done percutaneously and in short followup, there has been stabilization and even some reduction in the aneurysm circumference.
It was long known that a certain percentage of PTFE grafts “back in the day” would sweat ultrafiltrated plasma. The relative porosity of the grafts allowed for transudation of a protein rich fluid.

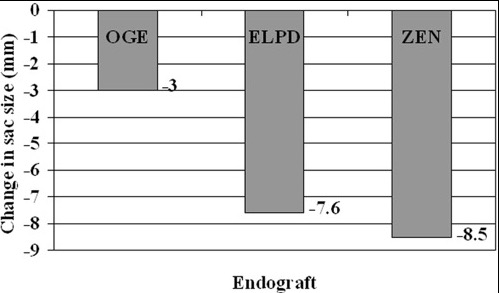
This results in a hygroma formation. I remember seeing this in AV graft fistulae back in the 90’s -after flow was introduced, the grafts would start sweating! The newer grafts are lower porosity and this is seen very infrequently. Drs. Morasch and Makaroun published a paper in 2006 comparing parallel series of patients who received the original Gore Excluder (OGE), the currently available Excluder Low-Permeability Device (ELPD), and the Zenith device (ZEN). Sac enlargement occurred in equal measure between OGE and ZEN but zero was reported for the ELPD.

The ELPD had higher rates of sac shrinkage than the OGE, and equal rates of sac shrinkage compared to ZEN.
The diagnosis in my patient’s case came about through serial followup through a decade. While I doubt that the aneurysm would have ruptured in the same way as in a Type I, II, or III endoleak, I am sure it would have progressed to developing symptoms from aneurysmal distension or local pelvic compression.
Is it possible to visualize this kind of endoleak at the time it is suspected? I came across a case series from the Netherlands using Gadofosveset trisodium which takes longer to clear than the usual Gd-based MR contrasts and they successfully visualized transudative leaks in 3 serial patients with the original Excluder graft.

The problem is that Gd-based contrasts have toxicity, especially for patients with poor renal function. The protocol is time consuming. And I suspect that ten years out, a lot of grafts will have positive findings, especially cloth based grafts that are sutured to their supporting stents, without clinical basis for treatment as their sacs size are likely stable on a year to year basis.
That said, as we are well into the second decade of commercially available stent grafts, it is even more important than ever to continue lifelong followup even for what is assumed stable, patent grafts and anatomy.